Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
Semaglutide is used therapeutically in two main ways:
Type 2 Diabetes Treatment: Semaglutide is marketed under brand names Ozempic and Rybelsus to help control blood sugar levels in people with type 2 diabetes. Semaglutide regulates insulin production, which helps lower blood sugar. Additionally, it reduces the amount of sugar produced by the liver. Ozempic is administered through a once-a-week injection. The starting dose is 0.25 mg per week, and it is adjusted every four weeks to 0.5 mg, 1 mg, and 2 mg. On the other hand, Rybelsus is an oral version of semglutide and is taken as a tablet once a day.
Weight Loss Treatment: Semaglutide is also approved under brand name Wegovy to help people with obesity lose weight. It influences appetite, making people feel less hungry, and it helps them eat less.
Similar to diabetes treatment, it's typically administered through a subcutaneous injection, usually on a weekly basis. The doses of semaglutide for weight loss (Wegovy) are different. The first month patients start with a dose of 0.25 mg, and increase to 0.5 mg after 4 weeks. In the third month the dose is increased to 1 mg, in the fourth month - 1.7 mg, and finally in the fifth month patients reach a maintenance dose of 2.4 mg.
Semaglutide products bear a black box warning.
A black box warning, also known as a boxed warning, is the most serious type of warning issued by the U.S. Food and Drug Administration (FDA) for prescription drugs. It is included in the prescribing information or labeling to alert healthcare professionals and patients about significant risks associated with the use of the drug.
Semaglutide boxed warning addresses the risk of thyroid C-cell tumors.
In rodents, semaglutide causes thyroid C-cell tumors at clinically relevant exposures. It is unknown whether semaglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined.
Semaglutide is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors.
In September 2023, the US Food and Drug Administration released updated safety information on semaglutide (Ozempic) products. The new labeling warns users about the heightened potential for ileus, a condition blocking the passage of food or liquid through the colon. Additionally, the updated label emphasizes that individuals using semaglutide in conjunction with an insulin secretagogue or insulin may face an elevated risk of hypoglycemia, including severe cases.
STEP 1: Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity
This study looked at whether a once-weekly injection of semaglutide, at a dose of 2.4 mg, could help adults lose weight when combined with lifestyle changes.
Nearly 2000 adults with a BMI of 30 or higher (or 27 or higher with certain weight-related conditions) were enrolled. They were randomly assigned to receive either once-weekly semaglutide or a placebo for 68 weeks, along with lifestyle changes. The main goals were to see how much weight people lost and how many achieved a weight reduction of at least 5%.
People taking semaglutide lost significantly more weight than those on the placebo - about 15% versus 2.4% at week 68. More semaglutide users achieved weight reductions of 5% (86.4% vs. 31.5%), 10% (69.1% vs. 12.0%), and 15% (50.5% vs. 4.9%) compared to the placebo group. The semaglutide group also showed better improvement in heart and metabolic health and reported feeling physically better. Common side effects were mild nausea and diarrhea, and more people in the semaglutide group stopped treatment due to stomach issues.
Conclusions:
For individuals with overweight or obesity, a weekly injection of 2.4 mg semaglutide, along with lifestyle changes, led to a significant and lasting reduction in body weight.
Source: https://clinicaltrials.gov/study/NCT03548935
STEP 2: Research Study Investigating How Well Semaglutide Works in People With Type 2 Diabetes Suffering From Overweight or Obesity
This study investigated the effectiveness and safety of once-weekly subcutaneous semaglutide 2.4 mg compared to semaglutide 1.0 mg (a dose approved for diabetes treatment) and a placebo for managing weight in adults with both overweight or obesity and type 2 diabetes.
This study involved adults from various regions, randomly assigned to receive semaglutide 2.4 mg, semaglutide 1.0 mg, or a placebo once a week for 68 weeks, along with lifestyle changes. The main goals were to see how much weight people lost and how many achieved a weight reduction of at least 5%. Safety was evaluated in all patients who received at least one dose of the study drug.
Among the 1210 participants analyzed, those receiving semaglutide 2.4 mg experienced a significant mean bodyweight reduction of -9.6% compared to -3.4% with placebo at week 68. At week 68, a higher percentage of patients on semaglutide 2.4 mg achieved weight reductions of at least 5% compared to the placebo group (68.8% vs. 28.5%). Adverse events were more frequent with semaglutide 2.4 mg and 1.0 mg compared to placebo, with gastrointestinal events being the most common.
In adults with overweight or obesity and type 2 diabetes, once-weekly semaglutide 2.4 mg demonstrated a superior and clinically meaningful decrease in body weight compared to a placebo.
Source: https://clinicaltrials.gov/study/NCT03552757
STEP 3: Research Study to Look at How Well Semaglutide is at Lowering Weight When Taken Together With an Intensive Lifestyle Program
The STEP 3 study aimed to compare the effectiveness of once-weekly semaglutide (2.4 mg) versus a placebo for weight management in adults with overweight or obesity. The treatment was combined with intensive behavioral therapy and an initial low-calorie diet.
This 68-week, double-blind study included 611 adults without diabetes, with overweight or obesity. Participants were randomly assigned to receive either semaglutide or a placebo, both with a low-calorie diet for the first 8 weeks and intensive behavioral therapy throughout the 68 weeks.
At the end of 68 weeks, those on semaglutide showed a significant weight loss of -16.0% compared to -5.7% in the placebo group. More participants on semaglutide achieved a weight loss of at least 5% (86.6% vs. 47.6%). A higher proportion of semaglutide participants also achieved weight losses of at least 10% or 15% compared to the placebo group. Gastrointestinal side effects were more common with semaglutide.
Conclusion:
For adults with overweight or obesity, once-weekly semaglutide, combined with intensive behavioral therapy and a low-calorie diet, resulted in greater and meaningful weight loss over 68 weeks compared to a placebo. Long-term effects need further exploration.
Source: https://clinicaltrials.gov/study/NCT03611582
STEP 4: Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity
The study aimed to understand whether continuing treatment with semaglutide, a medication for weight loss, is more effective than switching to a placebo in maintaining weight loss in individuals with overweight or obesity.
The research compared the effects of continued once-weekly treatment with semaglutide (2.4 mg) versus switching to a placebo for weight maintenance, both accompanied by lifestyle intervention. The participants had completed a 20-week initial period with semaglutide.
Conducted as a randomized, double-blind, 68-week phase 3a withdrawal study at 73 sites in 10 countries, the trial included adults with a body mass index (BMI) of at least 30 (or ≥27 with ≥1 weight-related comorbidity) without diabetes.
Participants received once-weekly subcutaneous semaglutide during a 20-week run-in period. After reaching the 2.4-mg maintenance dose, 803 participants were randomized (2:1) to 48 weeks of continued semaglutide or switched to a placebo, both with lifestyle intervention.
The primary endpoint was the percentage change in body weight from week 20 to week 68. Secondary endpoints included changes in waist circumference, systolic blood pressure, and physical functioning.
Of the 803 participants who completed the run-in period, those who continued semaglutide showed a mean body weight change of -7.9% compared to +6.9% for the placebo group. Waist circumference, systolic blood pressure, and physical functioning also improved with continued semaglutide. Gastrointestinal events were more common with continued semaglutide, and a similar proportion discontinued treatment in both groups due to adverse events.
Conclusion:
In adults with overweight or obesity, maintaining treatment with once-weekly semaglutide resulted in continued weight loss over 48 weeks compared to switching to a placebo after a 20-week run-in period.
Source: https://clinicaltrials.gov/study/NCT03548987
STEP 5: Two-year Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity
The STEP 5 trial aimed to assess the effectiveness and safety of once-weekly semaglutide 2.4 mg versus a placebo, both combined with behavioral intervention, for the long-term treatment of adults with obesity or overweight with at least one weight-related condition, excluding diabetes.
Conducted as a randomized trial from October 2018 to February 2019, 304 participants were assigned to either semaglutide 2.4 mg or a placebo. The majority of participants were female (77.6%), white (93.1%), with an average age of 47.3 years, a body mass index of 38.5 kg/m², and a weight of 106.0 kg.
The primary goals were the percentage change in body weight and achieving weight loss of ≥5% at week 104. The study assessed efficacy among all participants, regardless of treatment discontinuation or rescue intervention.
At week 104, the semaglutide group demonstrated a mean weight loss of -15.2% compared to -2.6% with the placebo. More participants in the semaglutide group achieved weight loss of ≥5% compared to the placebo group (77.1% vs. 34.4%). Gastrointestinal adverse events, mostly mild-to-moderate, were reported more frequently with semaglutide than with placebo (82.2% vs. 53.9%).
Conclusion:
In adults with overweight or obesity (with at least one weight-related condition), semaglutide treatment resulted in significant and sustained weight loss over 104 weeks compared to a placebo, with manageable gastrointestinal side effects.
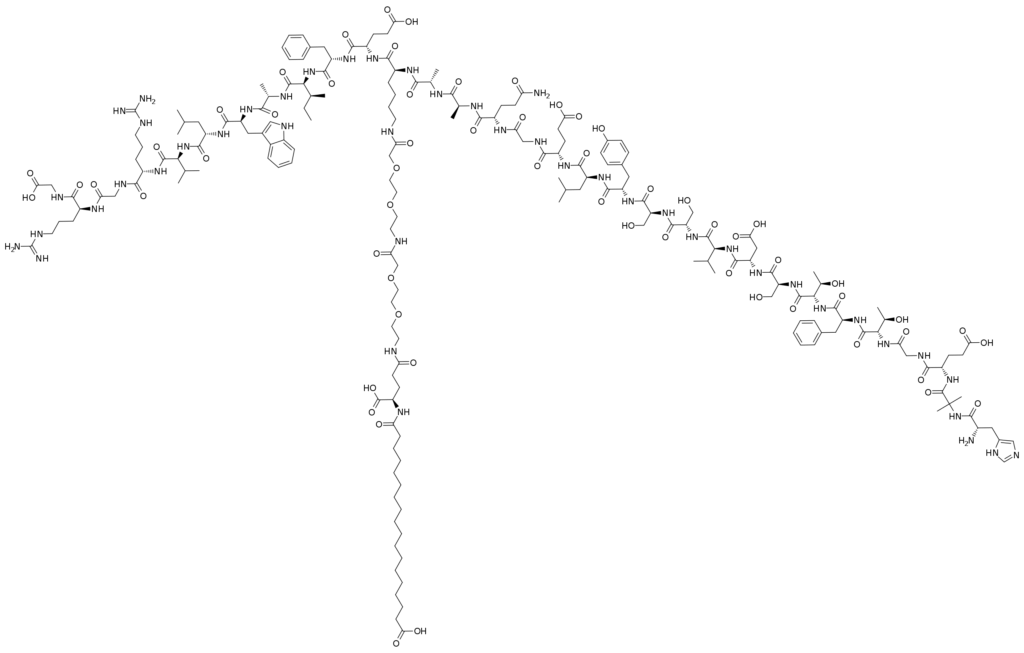
HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR
C187H291N45O59
910463-68-2
GLP-1, Wegovy, Ozempic, Rybelsus, NN9535
To ensure the highest quality of our products, we are committed to purity, validated through thorough laboratory testing. Each batch of semaglutide undergoes meticulous analysis in an independent laboratory. The purity of our products exceeds 99%
You can read our Shipping, Returns and Refunds Policy here.
We include a complimentary vial of bacteriostatic water and syringes for reconstitution to the orders over $200.
If you order multiple vials you may get a volume discount.
Orders over $400 have free shipping.
The products offered on this website are intended for research and development use only. The use of these products for human or animal consumption is strictly prohibited. The products offered on this website are not drugs, food, or cosmetics and should not be misbranded, misused, or mislabeled as such. These products are not approved by the U.S. Food and Drug Administration (FDA).
The products offered on this website are not intended to diagnose, treat, cure, or prevent any disease. They are sold solely for scientific and laboratory research purposes. Any improper use, including but not limited to the unauthorized handling, storage, or administration of these products, is the sole responsibility of the purchaser.
Sign up to be the first to know about new products, discounts and promotions.
While we make a reasonable effort to ensure the quality, accuracy, and safety of the content on our website, it is important to state that it is provided for general information purposes only and does not constitute medical advice. The content on this website, including text, images, and other content, is not intended to replace a consultation with an appropriately qualified medical practitioner.
SEMA.bio does not provide medical advice. Always consult your healthcare provider about possible interactions, contraindications, and allergies before starting any new supplement or medication.
Individual weight loss results may vary.
Products offered on this website are not approved by the U.S. Food and Drug Administration (FDA). These products are not intended to diagnose, treat, cure, or prevent any disease. Any improper use, including but not limited to the unauthorized handling, storage, or administration of these products, is the sole responsibility of the purchaser. This website and its product offerings are not associated, connected with or endorsed by Nоvо Nоrdіsk A/S in any manner.